Enantioselective Synthesis of Vicinal Diamines and β-Amino Amides by NiH-Catalyzed Hydroamidation of Alkenyl amides
By:Xun Tian; Shengzu Duan; Yuan Ma; Ailin Pan; Yamiao Meng; Guogang Deng; Hongbin Zhang; Xiaodong Yang
Organic Chemistry Frontiers
DIO: https://doi.org/10.1039/D4QO02275K
Published:2025-01-19
Abstract
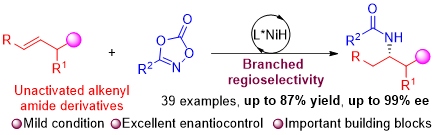
Enantioenriched diamines and β-amino amides are privileged scaffolds in organic and medicinal chemistry, which exhibit many applications in natural products and pharmaceutical molecules, as well as asymmetric catalysis. Catalytic asymmetric hydroamidation is considered one of the easiest ways to obtainsuch compounds in the enantioenriched form. Here, we report a NiH/BOX-catalyzed enantioselective hydroamidation of alkenyl amides with dioxazolones. The described transformation provides a series of enantioenriched vicinal diamines and β-amino amides, including structural modification of natural products and bioactive molecules and the preparation of the chiral radical scavenger nicaraven. A broad range of functional groups are well tolerated under room temperature with high enantioselectivities (up to 99%) and good yields (up to 87%). Mechanistic studies including the capture of a metal-nitrenoid intermediate and a competitive experiment provide significant evidence for this hydroamidation process.