Benzylic C(sp3)–H/C(sp3)–H Coupling with 2-Azaallyl Anions through Single-Electron Transfer and 1,5-Hydrogen Atom Transfer
By: Dongxiang Liu; Bijun Wang; Cuirong Qin; Haoqing Tang; Hui Li, Liang Li; Yonggang Jiang; Xiaodong Yang
Organic Letters
DIO: https://doi.org/10.1021/acs.orglett.5c00708
Published:2025-03-20
Abstract
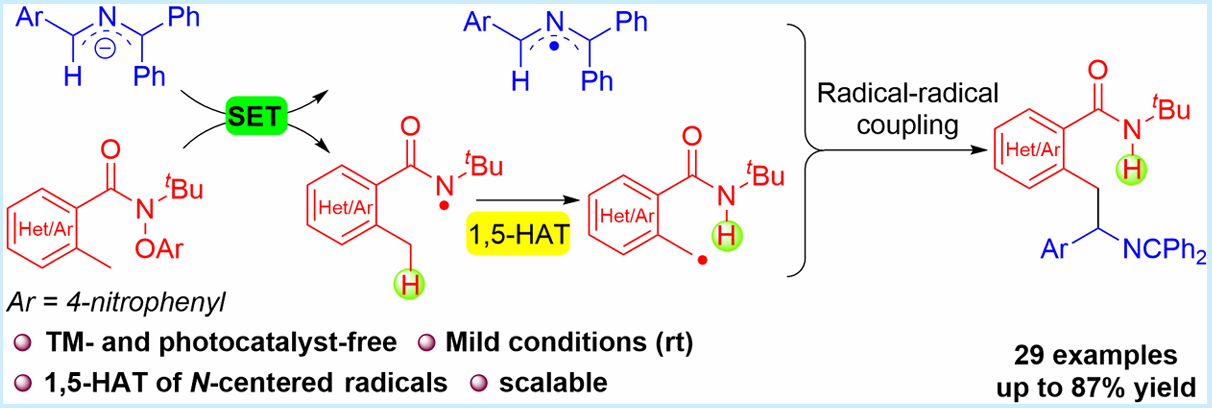
Herein is reported a novel transition-metal-free intermolecular C(sp3)–H/C(sp3)–H coupling of N-tert-butyl arylamides with N-benzyl imines through single-electron transfer (SET) and 1,5-hydrogen atom transfer (1,5-HAT) strategies. 2-Azaallyl anions as super-electron-donors (SEDs) undergo SET with N-tert-butyl arylamides to generate 2-azaallyl radicals and amidyl radicals. The amidyl radical undergoes a 1,5-HAT process to form a C-centered radical, which is subsequently coupled to a 2-azaallyl radical to generate new C–C bonds. This method avoids the use of transition metals and photoredox catalysts with good functional group tolerance and yields (29 examples, 87% yield). Radical clock and radical trapping experiments provide significant evidence for the 1,5-HAT process of amidyl radicals.