Nickel-Catalyzed Regio- and Enantioselective Hydroalkenylation of Aldehydes with 2-Azadienes
By: Ya Du; Lening Zhang; Shengzu Duan; Xiang Yang; Sina Liu; Hongbin Zhang; Patrick J. Walsh; Xiaodong Yang
Journal of the American Chemical Society
DIO: https://doi.org/10.1021/jacs.5c08602
Published:2025-08-07
Abstract
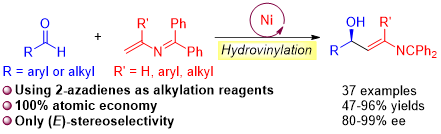
The development of new methods for the utilization of aldehydes has garnered much attention because their addition products, chiral alcohols, are common structural elements in the pharmaceutical industry. Despite significant progress in the catalytic asymmetric synthesis of secondary allylic alcohols, the synthesis of derivatives bearing amino functionalities remains challenging. Herein, we report a highly regio- and enantioselective nickel-catalyzed direct hydrovinylation of aldehydes with 2-azadienes, delivering a series of 2-azadiene-substituted alcohols in good yields (up to 96%) with high enantioselectivities (up to 99%) under mild conditions. These high-value γ-amino alcohols can be incorporated into natural products and bioactive molecules and are readily transformed into a variety of useful structural motifs.