Synthesis of Aminoalkyl Nitriles through 2-Azaallyl Anions-Driven Cascade Radical Ring-Opening/Intermolecular Coupling
By: Yonggang Jiang; Cuirong Qin; Haoqing Tang; Liang Li; Hongbin Zhang; Xiaodong Yang
Organic Chemistry Frontiers
DIO: https://doi.org/10.1039/D5QO01353D
Published:2025-10-16
Abstract
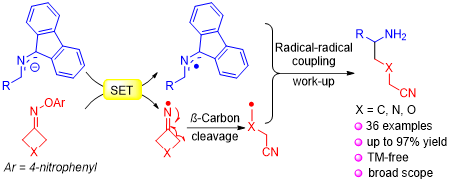
Aminoalkyl nitriles are common structural motifs in bioactive molecules and they serve as valuable intermediates in synthesis. The preparation of such moieties from radical cascade reactions, however, is rare. Herein is reported the first tandem single-electron transfer (SET)/ring-opening/intermolecular coupling between 2-azaallyl anions and cyclobutanone oxime ethers for the synthesis of aminoalkyl nitrile derivatives. Remarkable features of this method include mild reaction conditions, easy operation, and broad substrate scope (36 examples, up to 97% yield). Gram-scale synthesis and product derivatizations (δ-amino carboxylic acids, 1,5-diamines, lactams, lactam oximes) demonstrate the scalability and potential synthetic value of this approach. Mechanistic studies including EPR, radical trapping and radical clock experiments suggest that the reaction through a unique radical ring-opening/intermolecular coupling cascade mechanism.