Palladium-Catalysed Aminocarbonylation of Aryl Thianthrenium Salts to Aryl Carboxamides Using Mo(CO)6 as a CO Source
By: Zhuo Chen, Hui-Qiang Li, Shun-Bin Xu, Ling-Rui Zhou, Tong-Ling Qin, Wen-Xia Hu, Tao Li, Ruo-Yi Hou, Zi-Yu Lin and E Tang
Organic Chemistry Frontiers
DIO: 10.1039/d5qo01508a
Published:2025-12-30
Abstract
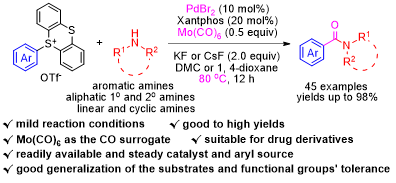
An efficient palladium-catalysed aminocarbonylation reaction between aryl thianthrenium salts and amines, using Mo(CO)6 as a solid CO surrogate, has been developed. A wide range of aliphatic and aryl carboxamides, including secondary and tertiary amides, as well as N-aliphatic-linear amides, N-aliphaticcyclic amides, and N-aryl amides, and some carboxamide derivatives of bioactive molecules such as loratadine, fenofibrate, and cloquintocet-mexyl, have been synthesized under safe and mild conditions and with good to excellent yields and broad functional group tolerance, including the bromo and iodo groups, offering a new avenue for the production of aryl carboxamides with potential applications.